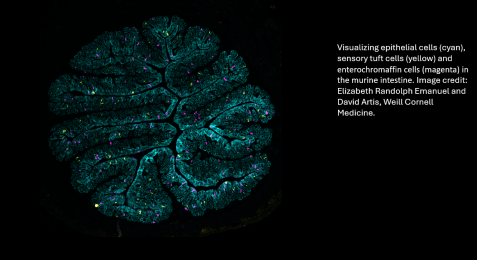
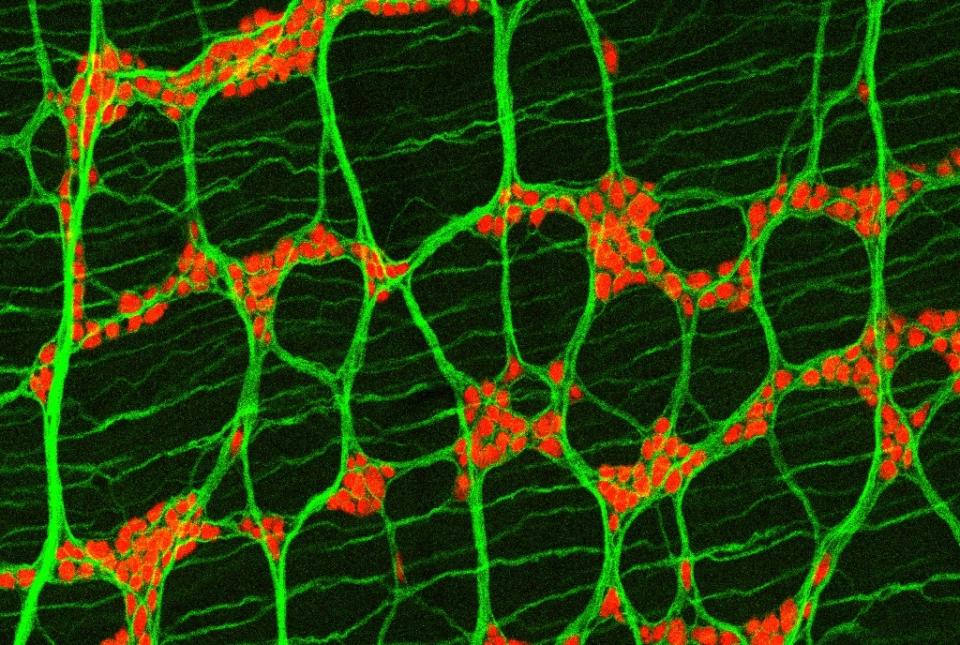
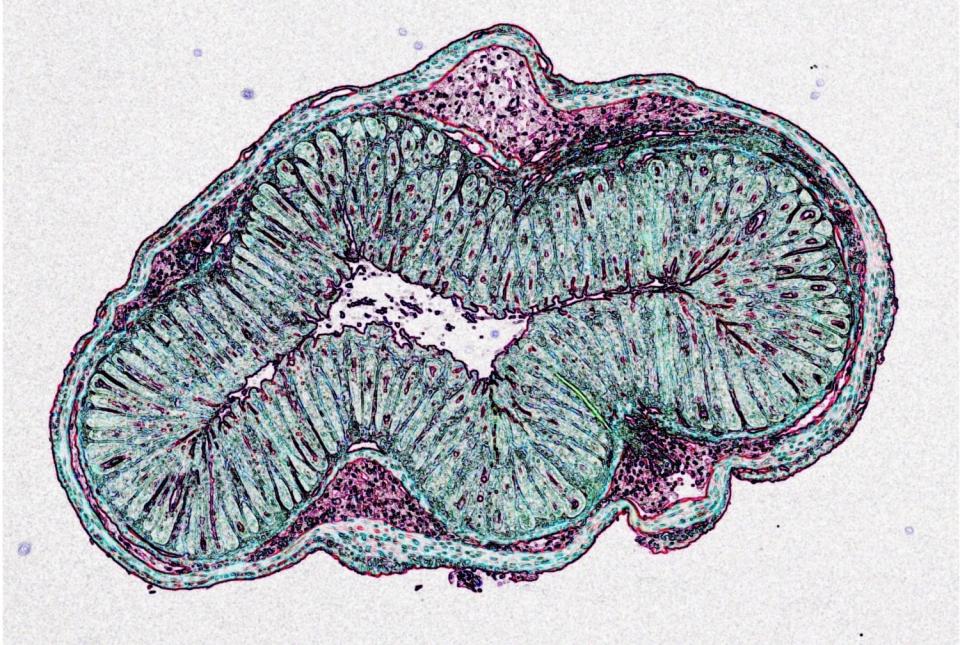
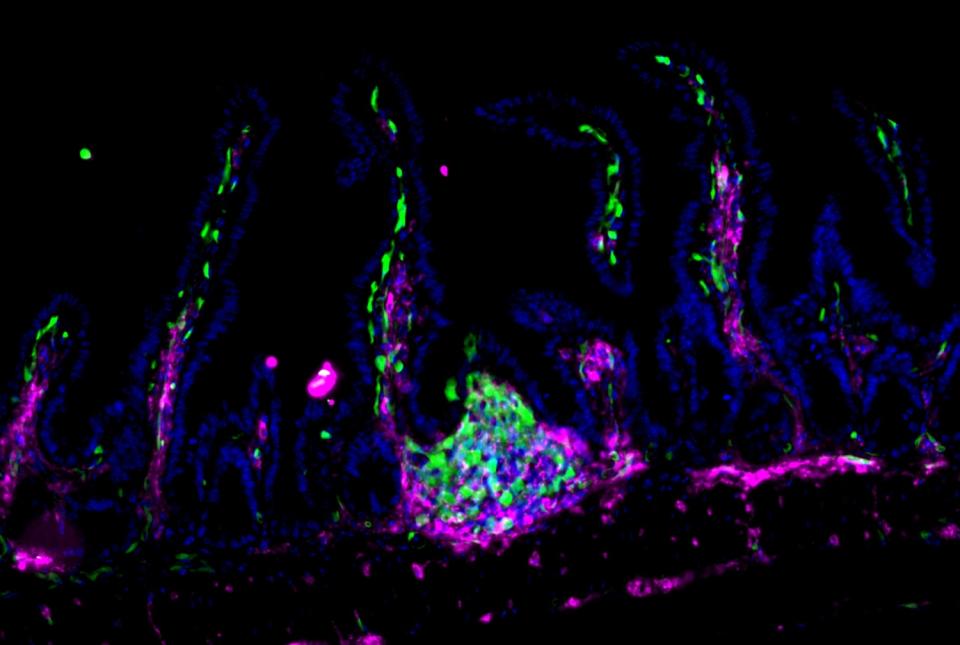
A group of immune proteins called the inflammasome can help prevent blood stem cells from becoming malignant by removing certain receptors from their surfaces and blocking cancer gene activity, according to a preclinical study by Weill Cornell Medicine investigators.
The study, published Jan. 2 in Nature Immunology, may lead to therapies that target the earliest stages of cancer. The findings bolster the idea that the inflammasome has a dual role—it promotes inflammation associated with poor outcomes in late cancer stages, but early on, it can help prevent cells from becoming cancerous in the first place.

Dr. Julie Magarian Blander
“What was striking was that the innate immune system, which includes the inflammasome, has a role beyond infection,” said Dr. Julie Magarian Blander, the Gladys and Roland Harriman Professor of Immunology in Medicine and a member of the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine. “We found that it functions in maintaining homeostasis in the tissue, keeping an eye on whether stem cells are proliferating too much. By doing so, it prevents cells from becoming cancerous and this activity is independent of inflammation.”
The co-first authors of the study are Dr. Andrew Kent, an assistant professor of medicine-hematology at the University of Colorado School of Medicine and Dr. Kristel Joy Yee Mon, a postdoctoral associate in Dr. Blander’s lab.
Origin Story
By the time patients typically go to the doctor with cancer symptoms, tumors have already formed. As a result, very little is known about cancer’s beginnings.
To get a better understanding of how the disease takes hold, Dr. Blander and her colleagues chose to study a mouse model of B-cell lymphoma called Eµ-myc, which has a mutation in the Myc oncogene. These mice have a long delay before tumors develop, giving researchers a chance to observe what happens early on in cancer. Because B-cell lymphoma develops in a type of white blood cell, the team examined their precursors, called hematopoietic stem cells, in the mice.
Genetically disrupting inflammasome activity in the Eµ-myc mice greatly accelerated stem cell proliferation and tumor development. The investigators were surprised to find that stem cells in control mice that lacked the inflammasome also proliferated at a fast pace compared with wild-type mice, suggesting that the complex has an important role in healthy cells, too. The team found that without the inflammasome, the stem cells have high levels of the protein Ras, which is another oncogene product. This protein can work together with mutant Myc to drive cancer, so the inflammasome’s normal job of keeping Ras in check delays tumorigenesis.
Ground zero for the protective activity was not the hematopoietic stem cells themselves, but the bone marrow stroma, a collection of many cell types surrounding and nurturing the stem cells.
Higher levels of soluble tumor necrosis factor (TNF) receptors were found in the stroma of control mice compared with the inflammasome-deficient mice. “It turned out that TNF receptors were being shed from stem cells in control mice, and they were being retained on stem cells from inflammasome-deficient mice. Higher TNF receptor levels lead to increased stem cell proliferation. Maintaining a healthy level of TNF receptors becomes important for these stem cells to maintain homeostatic control of proliferation,” said Dr. Blander. “We think that the inflammasome in the stroma is orchestrating something where it’s cleaving TNF receptors, shaving them off the stem cells.”
Next steps
Next, the team will test for the inflammasome’s protective effects in other tissues. In addition, they will determine which of the stromal cell types is responsible for the activity, and which molecules the inflammasome is using to suppress cell growth.
Ultimately, the researchers hope that the study will lay the groundwork for a therapeutic that would stave off cancer. “A therapy could target the inflammasome, but it should be directed only to the inflammation side of its activity that is associated with tumor progression,” said Dr. Blander, who is also a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine. “You want to protect the inflammasome’s beneficial function of delaying tumorigenesis.”
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, please see the profile for Dr. Julie Magarian Blander.
The work reported in this story was supported in part by the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, through grant numbers AI170832, AI170897, AI159772, and AI178327. Additional support was provided by the Jill Roberts Institute for Research in IBD at Weill Cornell Medicine.